ASE Interface¶
[1]:
import rdkit2ase
The main functionality of rdkit2ase is to generate new structures from SMILES. For this purpose, you can utilize smiles2atoms and smiles2conformers
[2]:
water = rdkit2ase.smiles2atoms("O")
water
[2]:
Atoms(symbols='OH2', pbc=False)
[3]:
aspirin = rdkit2ase.smiles2conformers("CC(=O)OC1=CC=CC=C1C(=O)O", numConfs=3)
aspirin
[3]:
[Atoms(symbols='C2O2C7O2H8', pbc=False),
Atoms(symbols='C2O2C7O2H8', pbc=False),
Atoms(symbols='C2O2C7O2H8', pbc=False)]
Within rdkit2ase you can translate structures between ASE and rdkit.
[4]:
mol = rdkit2ase.ase2rdkit(aspirin[0])
print(mol)
mol
<rdkit.Chem.rdchem.Mol object at 0x13be21380>
[4]:
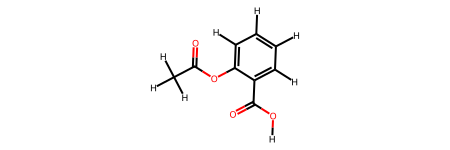
If a structure has been generated using rdkit2ase it includes smiles and connectivity in it’s atoms.info key. This information is utilized to quickly transform ase.Atoms to rdkit structures.
[5]:
aspirin[0].info
[5]:
{'smiles': 'CC(=O)OC1=CC=CC=C1C(=O)O',
'connectivity': [(0, 1, 1.0),
(1, 2, 2.0),
(1, 3, 1.0),
(3, 4, 1.0),
(4, 5, 1.5),
(5, 6, 1.5),
(6, 7, 1.5),
(7, 8, 1.5),
(8, 9, 1.5),
(9, 10, 1.0),
(10, 11, 2.0),
(10, 12, 1.0),
(9, 4, 1.5),
(0, 13, 1.0),
(0, 14, 1.0),
(0, 15, 1.0),
(5, 16, 1.0),
(6, 17, 1.0),
(7, 18, 1.0),
(8, 19, 1.0),
(12, 20, 1.0)]}
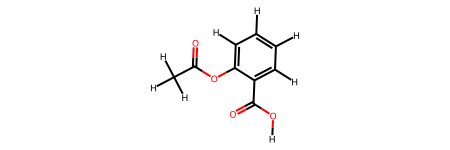
Without this information, rdkit2ase will try to guess the bond information. You can aid this process, by including SMILES into the suggestions= keyword.
[6]:
aspirin_0 = aspirin[0]
del aspirin_0.info["smiles"]
del aspirin_0.info["connectivity"]
rdkit2ase.ase2rdkit(aspirin_0, suggestions=[])
[6]:
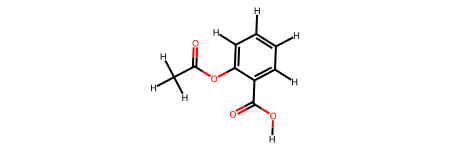
[7]:
rdkit2ase.ase2rdkit(aspirin_0, suggestions=["CC(=O)OC1=CC=CC=C1C(=O)O"])
[7]:
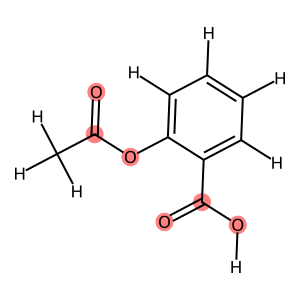
You can use match_substructure to select parts of the structure based on SMILES, SMARTS, molecules or even ase.Atoms.
[8]:
from rdkit.Chem import Draw
mol = rdkit2ase.ase2rdkit(aspirin_0, suggestions=["CC(=O)OC1=CC=CC=C1C(=O)O"])
Draw.MolToImage(
mol,
highlightAtoms={
i
for group in rdkit2ase.match_substructure(
aspirin[0], smarts="C(=O)O", suggestions=[]
)
for i in group
},
)
[8]:
This can also be employed for periodic structures. Even if they have crossed periodic boundary conditions.
[9]:
ethanol = rdkit2ase.smiles2conformers("CCO", numConfs=10)
box = rdkit2ase.pack([ethanol], [3], density=786, packmol="packmol.jl")
# let's move and wrap the box
box.positions += [2, 2, 2]
box.wrap()
[10]:
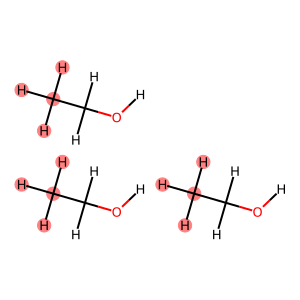
ch3_matches = rdkit2ase.match_substructure(box, smarts="[C]([H])([H])[H]")
print(f"Found {len(ch3_matches)} CH3 groups:")
for i, match in enumerate(ch3_matches):
print(f" CH3 group {i + 1}: atoms {match}")
mol = rdkit2ase.ase2rdkit(box)
Draw.MolToImage(
mol,
highlightAtoms={i for group in ch3_matches for i in group},
)
Found 3 CH3 groups:
CH3 group 1: atoms (0, 3, 4, 5)
CH3 group 2: atoms (9, 12, 13, 14)
CH3 group 3: atoms (18, 21, 22, 23)
[10]:
[ ]:
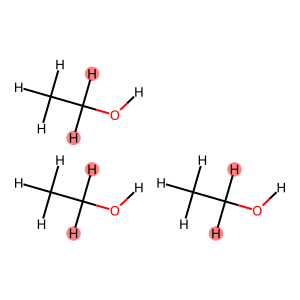
# 1. Define a general SMARTS pattern to find a CH2 group next to an oxygen.
# The pattern maps the two hydrogens as :1 and :2.
smarts_pattern = "[C;H2](-[H:1])(-[H:2])-[O]"
# 2. Perform the substructure search.
# The result 'ch2_hydrogens' will be a list of tuples.
# Each tuple contains indices for (Carbon, Hydrogen, Hydrogen, Oxygen).
# Example: [(4, 5, 6, 7)]
ch2_hydrogens = rdkit2ase.match_substructure(box, smarts=smarts_pattern)
# 3. Create an empty set to store only the hydrogen indices.
highlight_indices = set()
# 4. Loop through each match found.
for match in ch2_hydrogens:
# From each match tuple, extract the indices for the two hydrogens.
# These are at index positions 1 and 2 because of the [H:1] and [H:2] maps.
highlight_indices.add(match[1])
highlight_indices.add(match[2])
# 5. Print the final set of unique hydrogen indices.
print(f"Hydrogen indices to highlight: {highlight_indices}")
# 6. Convert the ASE object to an RDKit molecule for visualization.
mol = rdkit2ase.ase2rdkit(box)
# 7. Generate an image of the molecule, highlighting only the desired atoms.
Draw.MolToImage(mol, highlightAtoms=list(highlight_indices))
Hydrogen indices to highlight: {6, 7, 15, 16, 24, 25}
[ ]: